
The Significance of Partikel Bermuatan Positif Yang Terdapat Dalam Inti Atom Adalah

As an expert blogger, I’ll delve into the fascinating world of positively charged particles found in the nucleus of an atom. Understanding these particles is crucial to unraveling the mysteries of atomic structure. Let’s explore the significance of these positively charged particles and their role in shaping the behavior of atoms.Positively charged particles within the atomic nucleus play a pivotal role in defining the characteristics of elements and their interactions. By shedding light on these fundamental building blocks of matter, we can gain valuable insights into the forces that govern the universe. Join me on this enlightening journey as we uncover the secrets of these positively charged particles and their impact on the world around us.
Partikel Bermuatan Positif Yang Terdapat Dalam Inti Atom Adalah

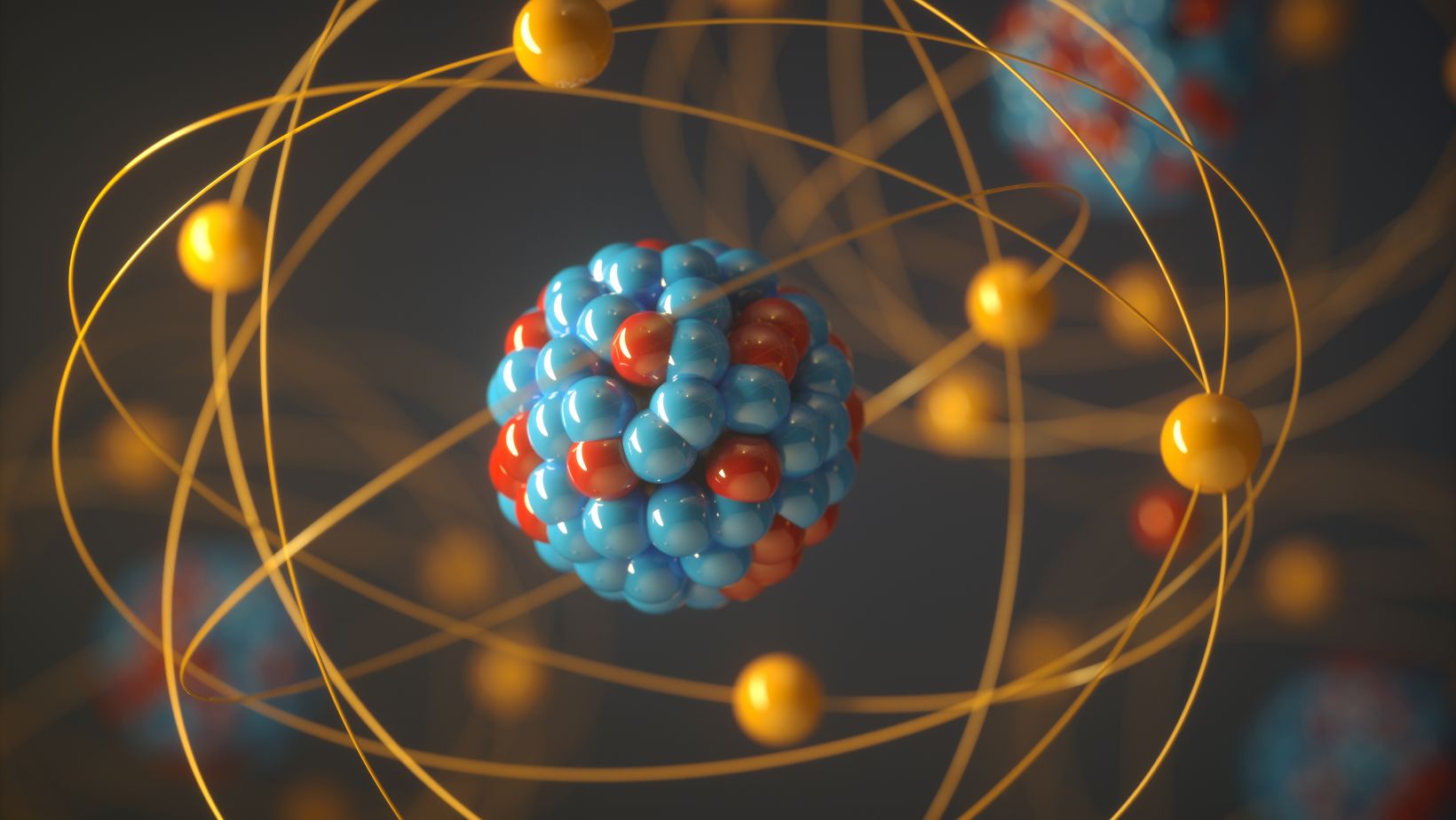
In the realm of atomic structure, Partikel Bermuatan Positif Yang Terdapat Dalam Inti Atom Adalah hold significant importance. These particles, also known as protons, play a crucial role in defining the characteristics of elements and their interactions. Understanding the nature of these positively charged entities provides valuable insights into the fundamental forces that govern the universe.Protons are not only integral to the composition of atoms but also contribute to the overall stability of matter. As one of the three fundamental subatomic particles, alongside neutrons and electrons, protons are essential building blocks of all elements in the periodic table. Their presence determines the atomic number of an element, distinguishing one element from another based on the number of protons it contains.The positively charged nature of protons also plays a key role in the formation of chemical bonds.
Positive Charged Particles in an Atom’s Nucleus

Positively charged particles within an atom’s nucleus are called protons. These particles play a fundamental role in defining the identity of elements. Protons are vital in determining the atomic number of an element, which distinguishes one element from another on the periodic table.In every atom, the number of protons is equal to the number of electrons, ensuring electrical neutrality. This balance between positively charged protons and negatively charged electrons maintains the stability and structure of the atom.The unique number of protons in an atom is what differentiates elements. For example, hydrogen has one proton, helium has two, and so on. Elements with differing numbers of protons exhibit different chemical properties, allowing for a vast array of compounds and interactions in the natural world.Protons, combined with neutrons, form the nucleus of an atom, creating a dense core that houses most of the atom’s mass.
Protons: The Building Blocks of an Atom
Definition of a Proton

A proton is a positively charged particle found in the nucleus of an atom. It plays a fundamental role in determining an element’s identity through its unique atomic number.
- Positive Charge: Protons carry a positive electrical charge.
- Mass: Protons have a mass of approximately 1.67 x 10^-27 kg.
- Stability: Protons contribute to the stability of an atom by interacting with neutrons in the nucleus.
- Atomic Number: The number of protons in an atom’s nucleus defines its atomic number, distinguishing one element from another.
Role of Protons in Atomic Structure

As I delve into the Partikel Bermuatan Positif Yang Terdapat Dalam Inti Atom Adalah, it’s evident that these positively charged particles are fundamental to understanding an element’s composition. Protons, located in the nucleus, define an element’s identity through its unique atomic number. Interacting with neutrons, protons contribute to atomic stability and distinguish one element from another on the periodic table.Protons, the positively charged particles found in an atom’s nucleus, are essential for defining an element’s identity. Their presence determines the unique atomic number of an element, distinguishing it from others on the periodic table. Working in harmony with neutrons, protons contribute to the stability of atomic structures. Understanding the significance of protons sheds light on the intricacies of atomic composition and the foundation of the elements we encounter in the universe.




